Abstract
Introduction: Without transplantation, survival of patients (pts) with relapsed acute myeloid leukemia (AML) after chemotherapy is poor. However, some pts may benefit from re-induction chemotherapy and enter a second or even third complete remission (CR).
Materials and methods: We identified 24 pts with de novo AML who showed a prolonged survival despite multiple relapses after intensive induction and consolidation chemotherapy (multiCR). These pts achieved repeated CR following multiple re-induction phases, each consisting of multiple courses of intensive poly-chemotherapy, including a first re-induction cycle (3+5+7, 3+7, MiDAC, or FLAG) followed by up to 4 consolidation cycles with intermediate-dose or high dose ARA-C (Sperr et al, Clin Cancer Res 2004;10:3965-3971; Sperr et al, Am J Hematol 2017;92:E567-E574). All pts entered CR for at least 3 times and/or had relapse-free intervals of at least 1 year. For comparison, 48 pts who achieved a long-term leukemia-free survival (LT-LFS) and 48 pts who failed to achieve CR despite intensive chemotherapy (non-responder, NR) were analyzed. These two control cohorts were matched for age, sex, and karyotype (according to SWOG criteria). For each multiCR patient two matched LT-LFS and two matched NR pts were selected. Using material stored in local biobanks next generation sequencing (NGS) for 72 molecular markers was performed in these pts.
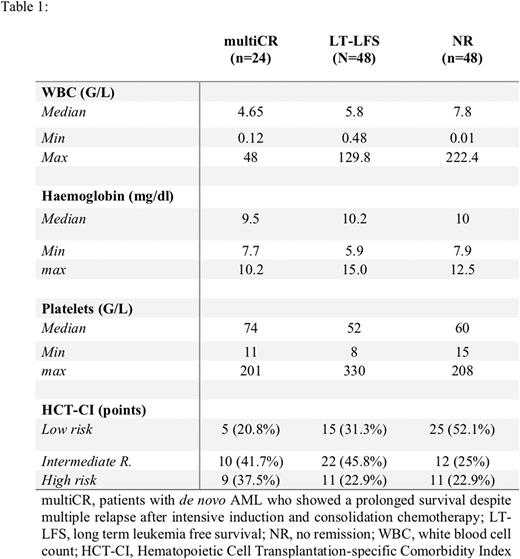
Results: The median age of the multiCR cohort was 64 (range: 38-76) years. At diagnosis, median white blood count (WBC) was 4.65 G/L in multiCR. No multiCR patient had a WBC >50 G/L. Fifteen pts (62.8%) presented with intermediate, 6 (25%) with unfavorable and 2 (8%) with a favorable karyotype as assessed by SWOG criteria. Comorbidities were detected in most pts. According to the Hematopoietic Cell Transplantation-specific Comorbidity Index, 5 pts (21%) were in the low, 10 (42%) in the intermediate, and 9 (37%) in the high-risk group. The characteristics of all pts´ groups are shown in table 1. In pts with multiCR the median continuous CR (CCR) was 2.3 years with a 95% confidence interval (CI) of 1.5-3.1 years. The median duration of the second and third CCR were 1.2 (95% CI: 0.9-1.4) years and 0.8 (95% CI: 0.4-1.2) years, respectively and thus significantly shorter compared to the first CCR (p<0.001). Of all pts, 21% achieved a 4th CR and 12.5% achieved a 5th CR. Significant differences in the CCR were found between multiCR and the LT-LFS cohort (multiCR median CCR 2.3 years; LT-LFS-cohort: median CCR not reached; p<0.001). The median overall survival of multiCR was 6.3 (95% CI: 5.2-7.3) years and differed significantly from the LT-LFS (15.5 years, 95% CI: 11.2-17.8 years) and NR (0.4 years, 95% CI: 0.3-0.5 years; p<0.001). By NGS mutations in 35 genes were detectable in all pts analyzed, with a median number of three mutated genes per patient. The median number of mutations per patient was 4 (range: 1-5) in multiCR, 3 (range: 0-5) in LT-LFS pts and 3.5 (range: 0-7) in NR. In the Multi-CR cohort FLT3-ITD (45.5%), BCOR (36.4%), IDH2 (36.4%), DNMT3A (27.3%), BCORL1 (27.3%), NRAS (18.2%), and STAG2(18.2%) were the most frequently mutated genes. In LT-LFS pts NPM1 (60.9%), TET2 (43.5%), DNMT3A (34.8%), FLT3-ITD (13.0%) and IDH2 (13.0%) had the highest prevalence. In those with NR FLT3-ITD (27.3%), NPM1 (27.3%), DNMT3A (22.7%), RUNX1 (22.7%), BCOR (22.7%), and ASXL1 (13.6%) were mutated most frequently. The MultiCRcohort differed markedly from the other groups with regard to the mutation in BCORL1, BCOR, FLT3-ITD and IDH2. Moreover, in contrast to LT-LFS and NR pts no multiCR patient was found to have a TET2 or a RUNX1 mutation (Figure 1).
Conclusions: Together, a small group of AML pts benefits from repeated re-inductions each consisting of multiple courses of intensive chemotherapy despite multiple relapses. Longer time intervals between first CR and relapse, and CR after one re-induction-cycle increase the likelihood that these pts will benefit from this therapy. These pts may also exhibit a typical NGS profile with a high prevalence of mutations in BCORL1, BCOR, FLT3-ITD and IDH2, and lack of mutations in TET2 and RUNX1. More studies and prospective evaluations in larger cohorts of AML pts are now required to confirm these data and to define the prognostic value of certain molecular landscapes in AML cells. Such evaluations may also lead to improved prognostication and personalized therapy in pts with relapsing de novo AML.
Disclosures
Meggendorfer:MLL Munich Leukemia Laboratory: Current Employment. Jaeger:Miltenyi Biomedicine: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Gilead: Honoraria; BMS Celgene: Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Honoraria; Sanofi: Honoraria; MSD: Honoraria; Beigene: Honoraria; Incyte: Honoraria; Acerta: Honoraria. Baer:MLL Munich Leukemia Laboratory: Current Employment. Haferlach:Munich Leukemia Laboratory: Current Employment, Other: Part ownership. Hoermann:MLL Munich Leukemia Laboratory: Current Employment. Valent:Blueprint Medicines: Honoraria; Celgene: Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Incyte: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal